Abstract
BACKGROUND: E4402 was a randomized phase III study comparing two different rituximab dosing strategies for patients with previously untreated, low tumor burden follicular lymphoma (FL). The primary endpoint was time to treatment failure. The initial publication (Kahl, JCO 2014) demonstrated that a retreatment strategy utilized less drug and produced comparable time to treatment failure compared to a maintenance strategy. Here we provide long term follow up results, focusing on response duration, time to first cytotoxic therapy, overall survival, and risk of histologic transformation.
METHODS: Eligible patients had untreated, low tumor burden (GELF criteria) FL. Patients received R 375 mg/m 2 weekly x 4 and responders were randomized to maintenance rituximab (MR) (single dose R q 3 months) or retreatment rituximab (RR) (R weekly x 4 doses at disease progression). Each strategy was continued until treatment failure. The primary endpoint, time to treatment failure, was defined as progression within 6 months of last R, no response to R retreatment, initiation of alternative therapy, or inability to complete protocol therapy. Secondary endpoints included time to first cytotoxic therapy, quality of life and safety. The ECOG Data Monitoring Committee recommended release of study results at a planned interim analysis in September 2011 and patients and providers were notified of results. Time to treatment failure data collection halted with release of the results but limited data collection on time to first cytotoxic therapy, response duration, and risk of histologic transformation continued.
INITITIAL RESULTS: From 11/03 to 9/08, 384 patients with FL were enrolled. Complete or partial response was achieved in 289 patients (71%), who were then randomized to MR (n=146) or RR (n=143). Demographic features were similar in the two arms: median age 59 years; ECOG PS 0-1 in all patients, and FLIPI low-risk (14.9 vs. 16.4%), intermediate-risk (46.3 vs. 42.9%) and high-risk (38.8 vs. 40.7%) for MR vs. RR, respectively. At initial publication, with a median follow-up of 3.8 years, the time to treatment failure was 3.9 years for MR vs. 3.6 years for RR (p=NS).
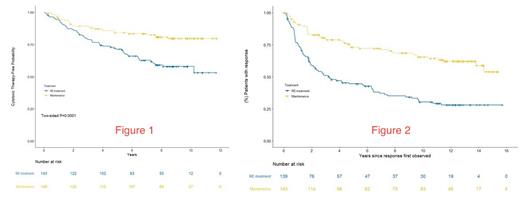
LONG TERM FOLLOW UP RESULTS: Immunoglobulin levels and risks for serious infections/late complications in MR patients will be updated at the annual meeting. For the endpoint of time to first cytotoxic therapy, the median follow up is 8.7 years. At 7 years, 83% of MR and 63% of RR remained free from first cytotoxic therapy (HR 2.37; 95% CI 1.50 - 3.76) [Figure 1]. For the endpoint of response duration, the median follow up is 12.1 years. At 10 years, 66% of the MR patients remained in their 1 st remission compared to 30% of the RR patients who remained in their 1 st remission [Figure 2]. The median response duration for RR patients receiving a single 4-week course of rituximab was 3.25 years. There was no difference in the overall survival at 10 years, 84% for MR vs. 83% for RR. There was a trend towards a lower risk of histologic transformation for patients receiving MR (n = 4) compared to RR (n = 11) (p = 0.11).
CONCLUSIONS: With long term follow up, the RESORT data indicates that in previously untreated, low tumor burden, follicular lymphoma, MR was superior to RR for delaying time to first cytotoxic therapy and for response duration, with a trend towards reducing the risk of histologic transformation. MR did not improve the overall survival. The original publication concluded that the time to treatment failure is similar between the two dosing strategies. Due to study design, time to treatment failure could not be analyzed in this long term follow up analysis. Compared to the historical benchmark of 3 years median time to first cytotoxic therapy when watch and wait is utilized, single agent rituximab, administered by either dosing strategy, was highly effective at delaying the time to first cytotoxic therapy.
Kahl: AbbVie, Adaptive, ADCT, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva: Consultancy; AbbVie, Acerta, ADCT, AstraZeneca, BeiGene, Genentech: Research Funding. Habermann: Incyte: Other: Scientific Advisory Board; Tess Therapeutics: Other: Data Monitoring Committee; Seagen: Other: Data Monitoring Committee; Morphosys: Other: Scientific Advisory Board; Loxo Oncology: Other: Scientific Advisory Board; Eli Lilly & Co.,: Other: Scientific Advisor. Schuster: Abbvie: Consultancy, Research Funding; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Research Funding; BeiGene: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; DTRM: Research Funding; Genetech: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Incyte: Research Funding; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; Merck: Research Funding; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Pharmaclcyclics: Research Funding; Tessa Theraputics: Consultancy; TG Theraputics: Research Funding. Fenske: TG Therapeutics: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy; Seattle Genetics: Speakers Bureau; Sanofi: Speakers Bureau; Pharmacyclics: Consultancy; MorphoSys: Consultancy; Kite (Gilead): Speakers Bureau; KaryoPharm: Consultancy; CSL Therapeutics: Consultancy; Bristol-Myers Squibb: Speakers Bureau; Biogen: Consultancy; Beigene: Consultancy; AstraZeneca: Speakers Bureau; ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Consultancy; AbbVie: Consultancy.